
In diagnostics, choosing the right antigen determines the difference between ambiguous results and clear, actionable insights. This is especially true for two critical markers:
- Native CRP (C-Reactive Protein)
- HBsAg Ad/Ay Antigen (Hepatitis B surface antigen subtypes)
Both play foundational roles in inflammation monitoring and hepatitis B screening.
In this blog, we will explain:
- Why Native CRP protein is still preferred in many diagnostics?
- The importance and challenges of HBsAg Ad/Ay antigen testing
- How deNOVO Biolabs offers validated, reliable solutions that support labs, CROs & diagnostic manufacturers globally? 🌍
1. Native CRP Protein:
What is CRP?
CRP is a liver-produced acute-phase protein that increases rapidly during inflammation or infection. Normal levels are below 1 mg/dL, while elevations into the tens or hundreds mg/L indicate significant inflammation or bacterial infection.
Why Native CRP is still widely used?
- Reflects authentic protein folding and modification as seen in vivo
- Maintains biological epitopes better than recombinant forms
- Favored for high-sensitivity inflammatory assays in clinical diagnostics
Clinical relevance:
Patients with CRP levels above 50 mg/dL are likely battling serious bacterial infections in about 90% of cases.
hs-CRP levels <3 mg/L can help assess cardiovascular risk in asymptomatic individuals.
Challenges in sourcing native CRP:
Extracting pure, clinical-grade CRP from human serum is logistically complex and constrained by donor availability but it is still considered the gold standard in many diagnostic workflows.
2. HBsAg Ad/Ay Antigens:
What is HBsAg Ad/Ay?
HBsAg detects infection with Hepatitis B virus (HBV). Subtype Ad and Ay relate to specific viral genotypes—crucial given global variability in HBV strains.
Why subtype matters?
Some geographic regions see predominance of Ad vs Ay subtypes
Subtype-specific antibodies yield higher sensitivity and specificity
HBsAg levels >0.1 IU/mL are considered positive, while <1 s/c is negative, helping differentiate latent vs acute infection.
Epidemiological context:
Approximately 257 million people globally live with chronic HBV, and up to 30% develop serious complications like cirrhosis or hepatocellular carcinoma annually making early detection vital.
Diagnostic performance:
Rapid diagnostic tests (RDTs) show sensitivities up to ~95% and specificity >98%, though accuracy dips in HIV-positive cohorts due to immune-complex interference.
3. Native CRP vs Recombinant CRP:
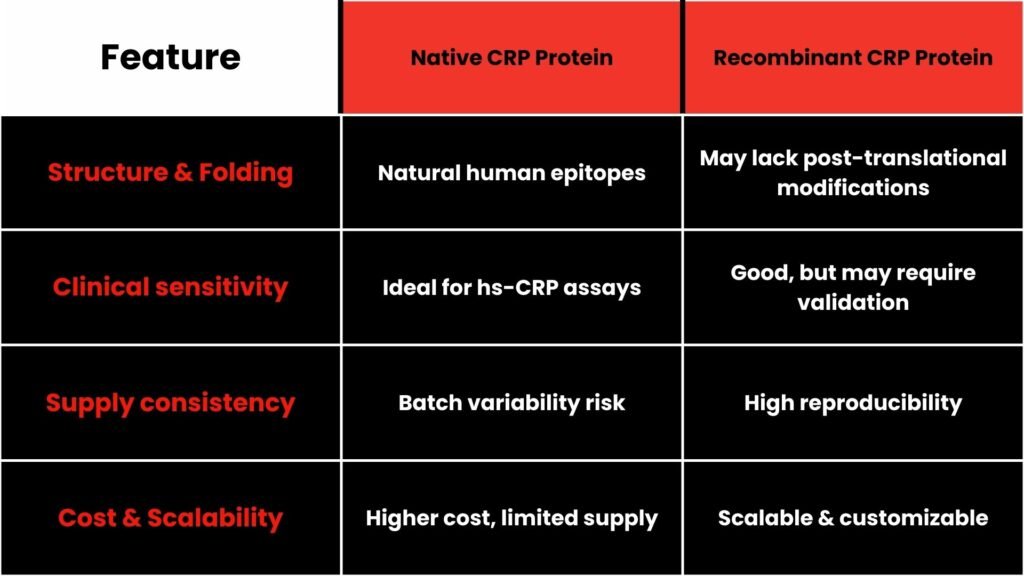
Native CRP remains preferable where clinical accuracy matters most while recombinant CRP suits scalable, standard research workflows.
4. Choosing the right HBsAg Ad/Ay Antigen products
Key decision criteria for diagnostic labs:
- Subtype matching (Ad vs Ay) for targeted regional accuracy
- Low detection limits (≤0.05 IU/mL) for early-stage screening
- Compatibility with ELISA, chemiluminescent, or LFA formats
Best practice:
Use antigens combined with international standard calibrators. When paired with neutralization and validation panels, subtype detection becomes significantly more reliable.
5. deNOVO Biolabs’ capabilities
Native CRP Protein:
- Extracted and purified to clinical-grade standards
- Supplied with certificate of analysis showing purity & activity
- Suitable for hs-CRP and inflammation monitoring assays
HBsAg Ad/Ay Antigens:
- Produced via recombinant engineering to mimic natural conformations
- Validated to subtype-specific panels and international reference standards
- Compatible with LFA rapid tests, ELISA, and chemiluminescent platforms
With deNOVO, labs and manufacturers get:
✅ High-purity antigens
✅ Lot-to-lot consistency
✅ Detailed QC documentation & batch traceability
6. Applications & Use Cases
Native CRP supports:
- Clinical labs monitoring infection or inflammation
- Hospitals tracking response to therapy or infection severity
HBsAg Ad/Ay supports:
- Diagnostic manufacturers launching subtype-specific kits
- Public health screening in endemic regions
- Vaccine or antiviral development monitoring
Using validated native CRP and subtype-specific HBsAg antigens helps minimize false negatives, especially early in disease detection.
7. Best Practices for diagnostic usage
- Always use validated calibrators & reference standards.
- Compare test performance across regional sample panels.
- Choose antigens compatible with your testing platform (LFA, ELISA, CLIA).
- Track batch performance with control charts and proficiency panels.
- Maintain transparent documentation for regulatory compliance.
8. Significance
As diagnostic testing becomes more decentralized and global, assay accuracy translates directly into better patient outcomes, earlier interventions, and fewer misdiagnoses. Both CRP and HBsAg are often cornerstones of screening panels for inflammatory diseases and viral hepatitis respectively.
Accurate antigen sourcing is not optional, it is the difference between credible diagnostics and unreliable results.
9. Why choose us?
At deNOVO, our goal is to provide dependable antigen solutions tailored for global diagnostic challenges.
Partner with us for:
- Clinically validated native CRP
- Subtype-specific HBsAg Ad and Ay antigens
- High QC standards with global traceability
- Expert technical support for assay development and validation
We help startups, CROs, and diagnostic manufacturers build assays that scale, reliably.
Are you developing inflammatory biomarker tests or hepatitis B diagnostics?
Let’s collaborate.
📩 Email: info@denovobiolabs.com
🌐 Visit: www.denovobiolabs.com
Together, we can ensure your diagnostics deliver accurate, reproducible results at scale.
