
The Backbone of Reliable Diagnostics
When clinical labs or diagnostic manufacturers deploy an assay, they trust that the reagents in #Batch A will behave the same as in #Batch B, months or years later. That expectation—that each batch of reagents, kits, antibodies, or substrates is consistent—is not just ideal; it is fundamental to diagnostic reliability, regulatory approval, and patient safety.
Yet, maintaining batch-to-batch consistency is a significant technical and operational challenge. In this blog, we will explore:
- Why consistency matters in diagnostics?
- Common sources of batch variability
- Methods & best practices to maintain consistency
- How deNOVO addresses consistency in its product lines?
- Real-world impact & a pathway for diagnostic teams
- How to partner with deNOVO to ensure consistency?
Why batch consistency is a ‘must’ in Diagnostics?
1. Accuracy & Reproducibility
A diagnostic test is only as trustworthy as its reagents. If one batch performs differently—slightly higher background noise, lower sensitivity, or altered kinetics—results shift, causing false positives or negatives, batch revalidation, or even recalls.
Imagine a hospital lab running COVID antigen tests: if one lot of substrate gives a weaker signal, borderline positive cases could be missed. Consistency ensures that thresholds remain stable.
2. Regulatory & Quality Expectations
Regulators expect critical quality attributes (CQAs) to be controlled. For diagnostic reagents, batch consistency is often implicitly required. customers, labs, and auditors expect lot-to-lot comparability. Variation beyond acceptable bounds invites questions and could block product approvals or acceptance.
3. Cost & Time Savings
Inconsistency leads to repeated validation runs, QC failures, and extra buffer lots. That means wasted material, time, resources. Ensuring consistency up front reduces downstream troubleshooting and accelerates time to market.
4. Brand Reputation & Trust
Diagnostic manufacturers depend on stable reagents. If your customers find that one batch gives different outcomes than another, they lose trust. Consistency is a promise you deliver—not just a technical checkbox.
Sources of Batch Variability
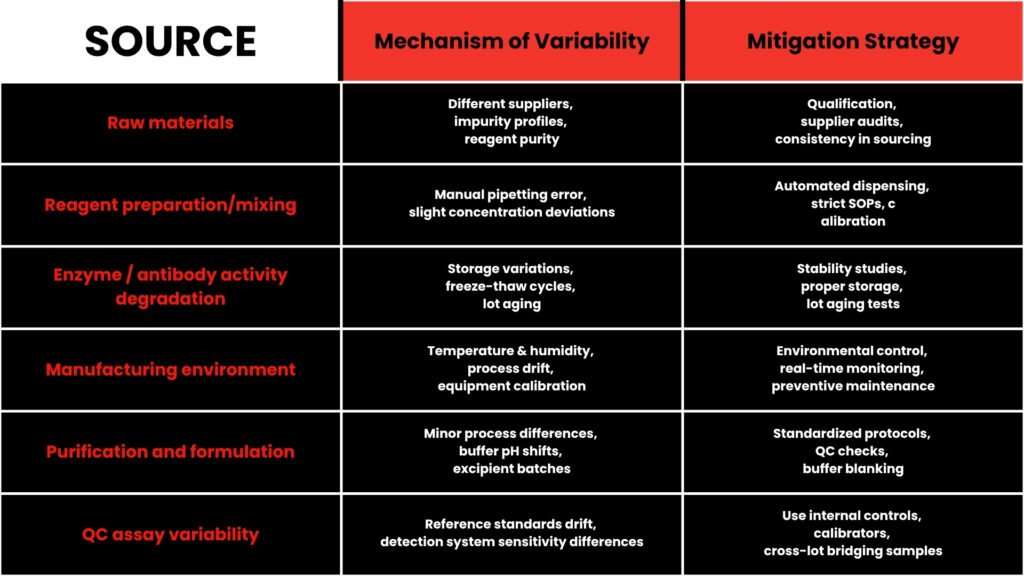
Scientific literature echoes the importance of controlling batch variation. For example, in process development, batch-to-batch variation often exceeds measurement error, making it a dominant source of process variation. (ScienceDirect)
In large-scale omics and proteomics studies, “batch effects” (systematic non-biological variation) are a recognized problem, affecting data reproducibility and downstream conclusions. (PMC)
Best Practices to ensure ‘consistency’
To manage batch consistency, diagnostic reagent makers should adopt a holistic approach:
Strict SOPs and protocols
Ensure every step—from mixing, incubation times, reagents volumes—is documented and adhered to precisely.
Calibration & equipment qualification
Instruments (pipettes, dispensers, incubators, spectrophotometers) must be regularly calibrated and validated.
Bridging & reference lots
Maintain a “golden lot” as a reference, and run bridging comparisons between new lot and reference to check drift.
Stability and shelf-life studies
Design accelerated aging tests, perform real-time monitoring, and set clear release criteria.
Control & QC samples
Use internal control samples across a dynamic range to flag batch shifts immediately.
Supplier consistency management
Prefer long-term suppliers or locked-in specifications, qualification studies, and incoming QC for raw reagents.
Lot-to-lot comparability metrics
Track key metrics (signal-to-noise ratio, limit of detection, background, kinetics) and set acceptable thresholds for variation.
Replication & statistical monitoring
Employ replicate runs, coefficient of variation (CV) metrics, and control charts to detect drifts over time.
How deNOVO approaches Consistency?
At deNOVO Biolabs, we built our product development philosophy around consistency:
- Batch Bridging:
Every new lot is compared to a reference, with side-by-side QC to confirm equivalence. - Stringent QC Standards:
For our reagents (ELISA kits, substrates, antibody pairs), we evaluate sensitivity, background, kinetics, and dynamic range. - Stability Protocols:
Accelerated aging, real-time stability, freeze-thaw cycle tests ensure that performance remains stable across the shelf life. - Controlled Sourcing:
We work with trusted suppliers and maintain audit trails for raw inputs to reduce upstream variability. - Robust Documentation:
Full traceability for every production batch, and transparency for customers who audit. - Responsive Support:
If a customer sees any drift, we investigate quickly—and supply bridging material or requalification help.
This systematic approach is one reason diagnostic manufacturers choose our reagents when they cannot compromise on reliability.
Relevance across applications

Challenges & Mitigation in Practice
Implementing consistency isn’t easy. Some challenges include:
- Process drift over time
- Rare but potent impurities evolving in minor amounts
- Changes in raw reagent landscapes (supplier discontinuations)
- Scale-up shifts when moving from small to large scale
Mitigation includes robust change control, continuous monitoring, frequent bridging, and contingency sourcing.
For teams building assays intended for clinics, hospitals, or decentralized testing, consistency is not a back-end issue — it is core to product promise. Diagnostic decision thresholds, result comparability across geographies, and regulatory acceptance all rely on reagents that behave the same across time and place.
If your diagnostic development or manufacturing team is evaluating reagent partners, ask about batch consistency protocols, bridging statistics and QC metrics. Do not accept vague assurances.
At deNOVO Biolabs, consistency is foundational to our design.
We invite you to:
- Request lot comparison data
- Test our bridging kits
- Collaborate for co-validation in your assays
Connect with us today to discuss how we can support your diagnostic pipeline with truly consistent reagents.
