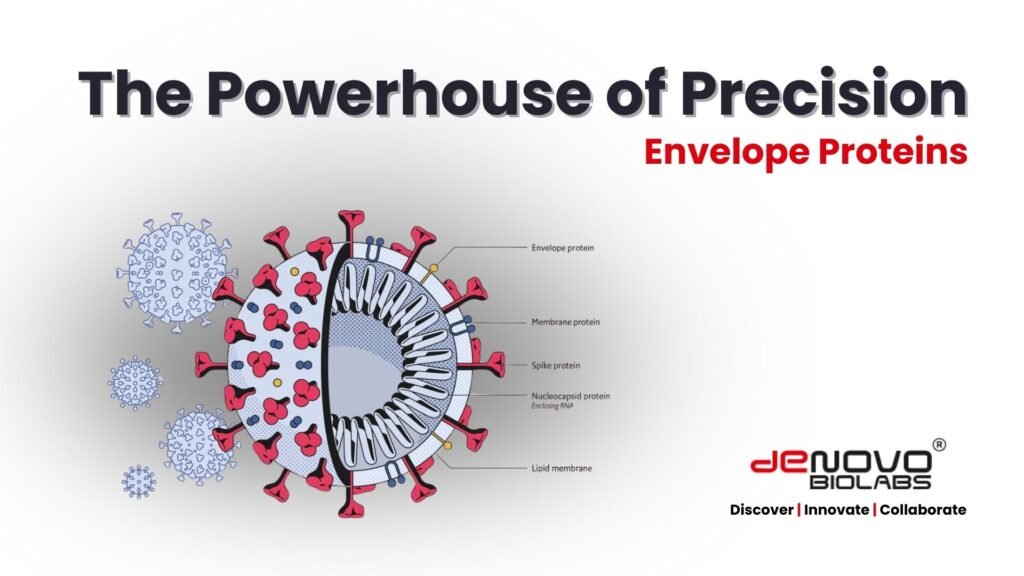
Envelope proteins are viral glycoproteins embedded in the outer membrane of enveloped viruses like coronavirus, influenza, HIV, and HCV. Such proteins like spike (S), envelope (E), and membrane (M) mediate host-cell entry and serve as primary targets for immune recognition.
Reagents based on viral envelope proteins are central to diagnostic tests, particularly in immunoassays, where high specificity and sensitivity are non-negotiable (Wikipedia).
In a landscape where viruses mutate rapidly, diagnostics built on envelope antigens can detect antibodies or antigens reliably—even across variants—helping combat emerging infectious threats with speed and accuracy (BioMed Central, BioMed Central).
Envelope Glycoproteins in Viral Diagnostics
1 Immune Recognition and Serological Testing
Host immune systems generate antibodies against viral envelope glycoproteins like gp120/gp160 in HIV or spike proteins in coronaviruses. Serological assays that use recombinant envelope proteins can detect these antibodies, enabling confirmation of past or active infection (BioMed Central, Nature).
For SARS-CoV-2, ELISA tests against spike (S) and nucleocapsid (N) proteins have demonstrated >92% sensitivity and 97–98% specificity (BioMed Central, Nature).
2. Antigen Detection in Rapid Tests
Envelope proteins can serve as direct antigens in lateral flow immunoassays (LFIA), offering fast and inexpensive point-of-care diagnostics. These tests are especially important in detecting active infection stages, aiding in outbreak control and epidemiological surveillance (MDPI, arXiv).
Diagnostic formats using Envelope Proteins
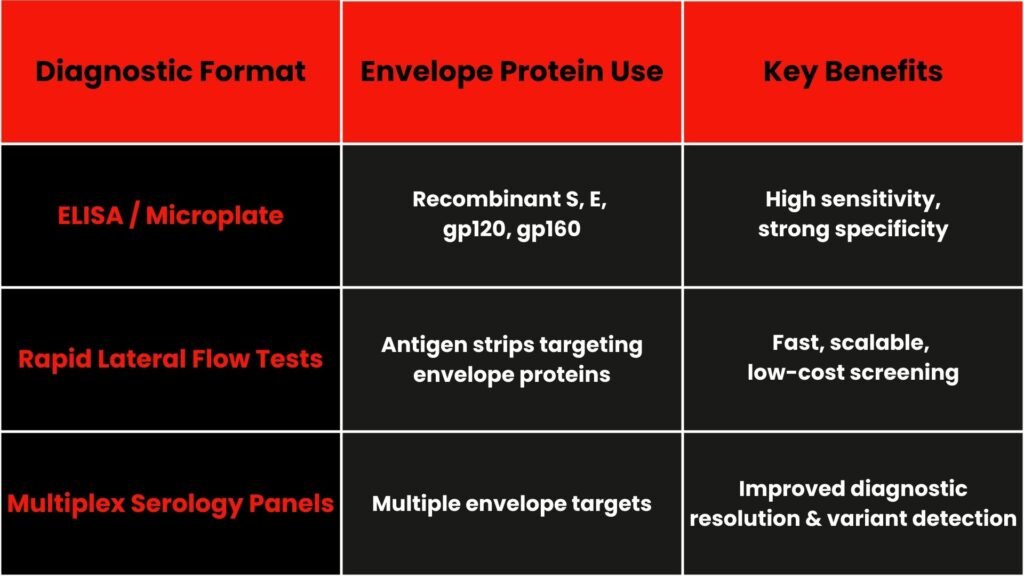
- ELISA assays: Standardized recombinant proteins offer reproducible standard curves and consistent calibration across batches
(PMC, BioMed Central). - LFIAs: Detected antigens directly from serum or nasal samples—helping contain infectious spread through early detection
(MDPI, arXiv). - Multiplex assays: Simultaneous detection of antibodies to S, E, M or related antigens can improve specificity in seroepidemiology and vaccine monitoring
(Nature, BioMed Central).
How deNOVO supports Envelope-Protein based IVD development?
At deNOVO Biolabs, we specialize in producing high-purity recombinant envelope antigens tailored for diagnostic assay development:
- Recombinant expression systems:
Using E. coli, CHO, or mammalian platforms to produce S, E or other viral proteins in correct folded, functional form. - Purity & stability:
≥95% purity, low endotoxin, consistent antigen conformation. Each lot comes with COA/TDS. - Functional validation:
Tested in ELISA calibration curves, antibody binding assays, and LFIA formats under real-sample conditions. - Custom formats:
Available with tags or specific isoforms to suit LFIA strips, microarray assays, or multiplex panels.
We also provide validated monoclonal antibody pairs for sandwich assay development—ideal for envelope antigens—and supply supporting reagents like substrates or buffers for enhanced detection.

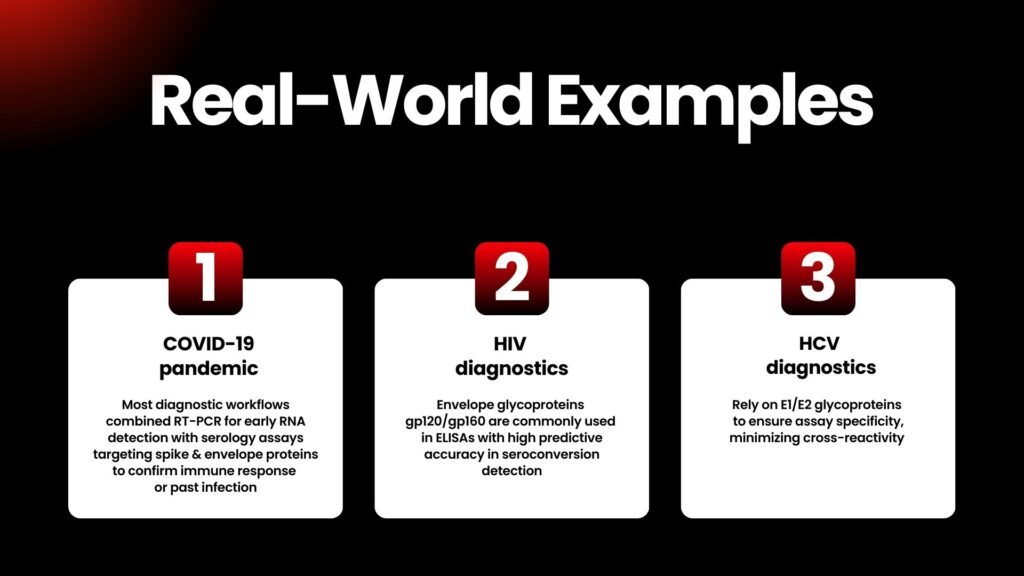
Future of Envelope Proteins & emerging diagnostics
As viruses evolve, envelope protein targets remain central to reliable diagnostics—even with mutations. Though genome-based tests like RT-PCR and CRISPR methods provide early detection, antigen and serological assays are crucial for scalable surveillance, vaccine efficacy monitoring, and adaptive diagnostics (Frontiers, Nature).
Advanced formats—like protein-based biosensors, CRISPR-linked lateral flow assays, or microfluidic ELISA—often rely on well-characterized envelope protein reagents for consistent performance (PMC, BioMed Central).
Tips for choosing Envelope Antigens
- Select full-length vs subunit based on assay sensitivity needs and cross-reactivity risk.
- Prioritize functionality—test electrophoretic profile, conformation, glycosylation status.
- Match antibodies precisely—use validated pairs for antigen detection.
- Include clinical sample validation in assay validation phase early.
Why choose us?
- Expertise in recombinant antigen and antibody reagent development for viral diagnostics.
- Quality-first approach: High purity, lot documentation, biological validation.
- Scalability: From pilot batches to large-scale production on demand.
- Technical support: From antigen selection through assay validation and regulatory submission.
Empower your diagnostic development with envelope-protein-based reagents you can trust.
Let deNOVO support your IVD innovation when you are building ELISAs, LFIAs, multiplex panels, or biosensors.
Contact us today to request a technical data sheet, sample panel, or application consultation.
🔖 References
- N. Bhattacharyya et al., “Viral glycoproteins: Biological role and application in diagnosis”… PMC VirusDisease 2016
(PMC, Wikipedia) - Envelope protein immunodiagnostics in emerging pathogens and pandemic viral detection pathways
(PMC, BioMed Central) - Role of envelope proteins in viral entry and immune susceptibility
(MDPI, Wikipedia)
