
Detecting neutralizing anti-etanercept antibodies is essential for accurate anti-drug antibody (ADA) and cell-based assays. These antibodies can directly negate the biologic activity of etanercept, affecting efficacy, pharmacokinetics, safety, and clinical outcomes. In this article, we explore why identifying neutralizing ADA matters especially for biopharma developers, CROs and diagnostic labs—and how deNOVO Biolabs offers validated tools for reliable testing.
Why Neutralizing Anti-Etanercept Antibodies matter?
Etanercept, a fusion protein targeting TNF-α, is widely used to manage autoimmune conditions. While clinical studies show lower ADA incidence compared to monoclonal TNF inhibitors, neutralizing antibodies can still emerge. Though anti-etanercept ADA are typically non-neutralizing (~18.5% incidence over 96 weeks) and not always linked to clinical loss of efficacy, their detection is critical when assessing biologic integrity and immunogenicity risk Actas Dermo-Sifiliográficas.
Neutralizing ADA can bind the active site or block TNF binding, potentially reducing drug levels and accelerating clearance. Without rigorous assays, these antibodies may go undetected, leading to misinterpretation of clinical trial or therapeutic performance.
ADA vs. Neutralizing ADA: What’s the Difference?
Binding ADA assays detect any antibody response regardless of function. In contrast, neutralizing antibody assays identify antibodies that directly block etanercept’s binding to TNF-α, impacting therapeutic function U.S. Food and Drug Administration.
For regulatory insight and clinical relevance:
- Binding ADA helps flag immunogenic potential.
- Neutralizing ADA impacts drug activity and patient outcomes.
Characterizing both types is vital for biologics development and post-market safety.
Cell-Based Assays:
The Gold Standard for Neutralization Testing
Functional or cell-based neutralization assays measure drug activity in the presence of patient serum. For anti-TNF biologics, these often use TNF-stimulated reporter cells to quantify inhibition of cytokine signaling U.S. Food and Drug Administration+1.
This real-world modality confirms whether ADA present in serum actually block etanercept’s pharmacodynamic function, providing actionable data on drug potency under immune pressure.
Clinical Impact of Neutralizing ADA
Studies of anti-TNF therapies reveal significant clinical consequences when neutralizing ADA are present. For instance, a real-world study on RA patients found nearly 0% ADA incidence with etanercept—versus ~24% among those treated with monoclonal antibodies like adalimumab—in whom neutralizing ADA correlated with lower drug levels and reduced remission rates PLOS.
Other research indicates up to 70% ADA incidence in some cohorts treated with adalimumab within 28 weeks, though not all are neutralizing Nature. These findings underscore the need for sensitive, drug-tolerant, and functional assays.
Why High Sensitivity & Drug Tolerance are important in ADA Testing?
Early ADA assays often underestimated incidence due to drug interference. Modern drug-tolerant platforms—like electrochemiluminescence (ECL) with immunomagnetic separation—detect both free and complexed antibodies, reaching sensitivity down to ng/ml ranges Frontiers+1.
High sensitivity ensures even low titer ADA are identified. Drug tolerance ensures ADA detection even when etanercept remains in the patient’s serum. Combining both is critical to understand clinical relevance—especially for neutralizing ADA.
deNOVO Biolabs’ Capabilities
At deNOVO Biolabs, we specialize in custom development and validation of neutralizing anti-etanercept antibody reagents for ADA and cell-based assays. Our capabilities include:
- Custom monoclonal reagents with controlled specificity and affinity
- Validated assay kits optimized for drug tolerance and sensitivity
- Cell-based neutralization panels, ideal for verifying ADA function
- Support for both preclinical and clinical assay validation
This ensures manufacturers and CROs get dependable tools to detect ADA accurately and protect biologic efficacy.
Use Cases & Applications
Diagnostics labs, CROs, and pharma developers use these tools to:
- Monitor immunogenicity during clinical trials
- Validate biosimilar interchangeability with etanercept
- Support regulatory submissions via robust ADA characterization
- Track immunogenic responses in post-market surveillance
Reliable neutralizing antibody detection helps mitigate risks, preserve drug activity, and reinforce confidence in biologic therapies.
Best Practices for ADA & Neutralizing Antibody Testing
- Use tiered testing:
Start with a sensitive ADA binding assay before confirming biological relevance with cell-based neutralization. - Ensure drug tolerance:
Select platforms designed to detect ADA in presence of therapeutic drug. - Quantify titers:
Higher ADA levels correlate more strongly with clinical impact than mere presence
U.S. Food and Drug Administration. - Track isotypes:
IgG types often dominate neutralizing responses. Understanding isotype helps interpret ADA persistence and affinity. - Regular sampling:
ADA often appear within weeks of therapy; longitudinal monitoring helps catch immunogenicity early.
Benefits of Accurate Neutralizing ADA Data
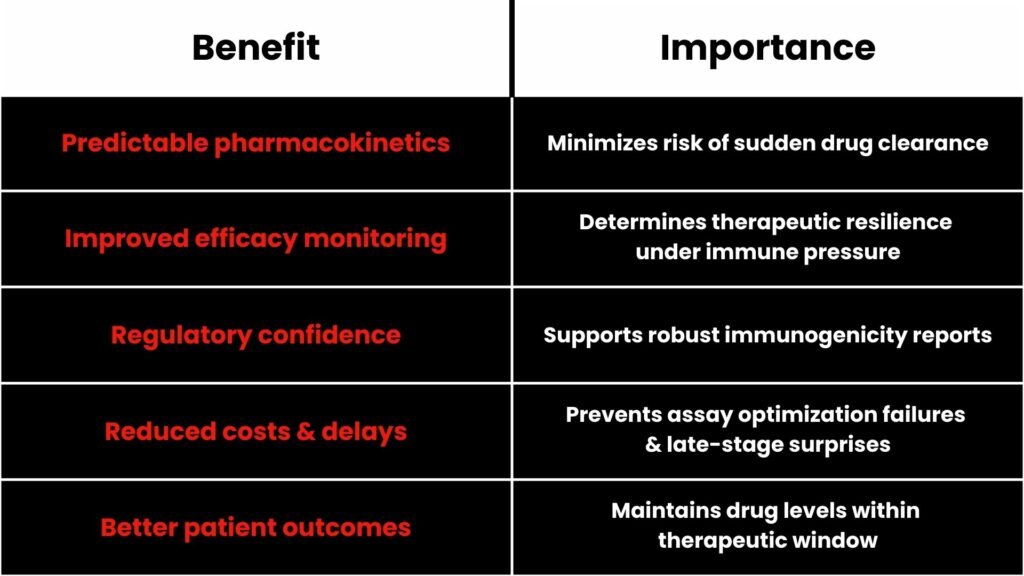
Essentially, functional ADA detection safeguards biologic investment and patient response.
Why partner with us
for ADA & Neutralizing Antibody Assays?
We deliver more than reagents—we provide trusted assay solutions developed with scientific rigor and user collaboration.
Our clients choose deNOVO because:
- We utilize cutting-edge drug-tolerant platforms
- We support custom assay design for specific drug profiles
- Our reagents offer batch consistency and global traceability
- We provide technical guidance for protocol validation and regulatory compliance
With deNOVO, ADA and NAB testing is not just accurate—it’s dependable.
If your team is working on etanercept biosimilars, TNF-inhibitor immunogenicity, or cell-based drug assays, let’s collaborate.
Explore neutralizing anti-etanercept antibody kits
Partner for custom assay design & validation
Contact us at info@denovobiolabs.com or visit denovobiolabs.com to learn how we can support your biologic testing needs 📩

binance odprt racun
16 Feb 2026Your article helped me a lot, is there any more related content? Thanks!