A smart checklist for CROs, Biopharma & Diagnostics
The biotechnology procurement landscape is rapidly evolving with a greater focus on quality, compliance and speed to market. If you are a CRO, diagnostic kit manufacturer, or a biopharma company working on biosimilars or novel therapies, your success hinges on sourcing the right reagents, kits, and services at the right time.
But with so many vendors and fluctuating quality standards, how do you choose a partner you can trust?
This Biotech Buyer’s Guide 2025 walks you through everything you need to evaluate while sourcing reagents, assay kits, antibodies, proteins, or custom lab services—and how Denovo Biolabs is building that very ecosystem.
Step 1
Align product selection with your regulatory path
RUO vs. IVD-grade makes a world of difference.
Step 2:
Ask for third-party validation or published studies to gauge real-world performance.
Step 3
Look for platform-specific guidance from the vendor—well-designed kits should feel like plug-and-play.
Step 4
Partner with a vendor that offers both pilot-scale flexibility and commercial-scale consistency.
Step 5
Ask if the vendor supports standing orders, scheduled dispatches, and emergency fulfilment.
Step 6
Support teams that include PhD-level scientists are a huge plus when optimising complex assays.
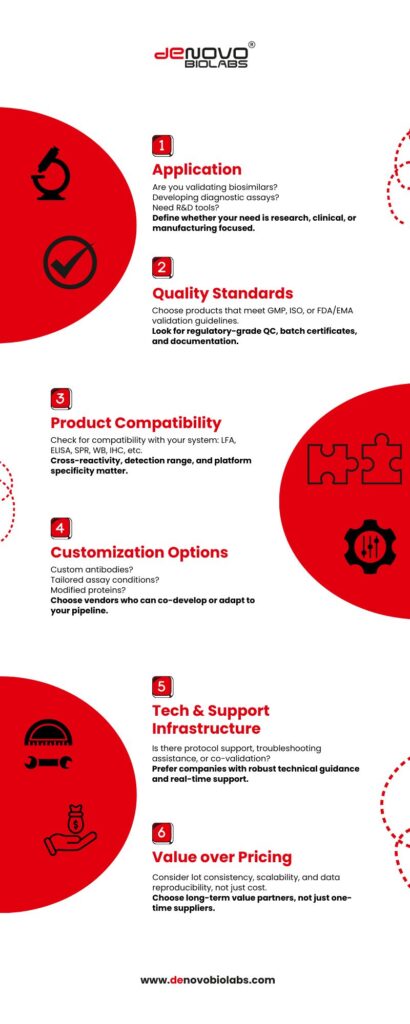
1: Define the Purpose
Is It Research, Diagnostic, or Regulatory?
Before selecting any biotech product or service, clarity of end-use is essential. Ask:
- Are you performing preclinical R&D, clinical assay development, or GMP-level QC?
- Are your kits for in vitro diagnostics, pharmacokinetic studies, or exploratory biomarker analysis?
- What compliance guidelines do you need to follow—FDA, EMA, CDSCO, NABL, or ISO 13485?
Why?
The grade of reagents, sensitivity of kits, and regulatory readiness of suppliers must match your intended purpose.
Why us?
We clearly mark all our products as “For Research Use Only” (RUO) or “Diagnostic Grade,s” depending on the validation level, use-case, and quality assurance protocols.
2: Ask for Product Performance Data
Data speaks louder than claims. Before purchasing, ensure your supplier provides:
- Validation reports with intra-/inter-assay CV%, recovery, and linearity
- Lot-to-lot consistency data
- Specificity & cross-reactivity profiles (especially for antibodies & ELISA kits)
- Publication support or third-party citations (where applicable)
Why us?
Our products come with validated technical datasheets, and our Adalimumab PK ELISA Kit was recently featured in a peer-reviewed journal for its performance in a clinical biosimilar study.
3: Choose the Right Partner for Antibodies & ELISA Kits
If you are sourcing:
- Primary or secondary antibodies → Verify species reactivity, labeling type (HRP, biotin), and assay compatibility.
- ELISA kits → Check detection limits, sample types (plasma/serum), and assay principles (sandwich, competitive, etc.).
⚠️ Beware of off-the-shelf solutions with vague specifications.
Why us?
We provide:
- Biosimilar PK ELISA kits validated for molecules like Adalimumab, Infliximab, etc.
- Research-grade ELISA kits for cytokines and immunological markers.
- Goat/Rabbit HRP-conjugated secondary antibodies with high purity & zero cross-reactivity.
4: Evaluate Customisation Capability
It can be a custom monoclonal antibody, a Sanger sequencing service, or a lateral flow assay for a novel target—you need a vendor that offers fit-for-purpose development.
Questions to ask:
- Do they offer antibody generation in rabbits/mice/goats?
- Can they label antigens or antibodies with enzymes/dyes?
- Will they sign NDAs or IP-safe contracts?
Why us?
We provide:
- Custom antibody development (monoclonal, polyclonal)
- LFA development for diagnostics
- Antigen-affinity purification
- Antibody/antigen labeling for various platforms
- Confidential, collaborative projects with full tech transfer options
5: Bulk Ordering?
Check for Scalability & Logistics
As your project grows, so does your need for batch consistency, inventory planning, and cold-chain logistics.
Ask your supplier:
- Can they deliver bulk lots with QC batch records?
- Do they maintain -20°C / 2–8°C logistics?
- How soon can they fulfill repeat orders?
Why us?
We ship all diagnostics and proteins in cold-chain compliant packaging and offer bulk & repeat-order support for diagnostic manufacturers and CROs.
6: Don’t Forget Support, Documentation & Service
It is not just the product, it is also the technical support, regulatory documentation, and expert advice that you need access to.
Look for:
- Responsive scientific support teams
- Validation protocols and user manuals
- Regulatory documentation for diagnostics
- Ongoing batch traceability
Why us?
We assign scientific consultants to key accounts and provide:
- Troubleshooting support
- Validation help for regulatory filings
- Documentation and product certifications where required
Final Thought
Biotech success in 2025 will be defined by collaboration, speed, and reliability. If you are developing a biosimilar, designing a diagnostic kit, or scaling immunoassays for CROs—partnering with a responsive, future-ready company is the difference between delays and breakthroughs.
Denovo Biolabs is helping leading biotech companies, CROs, and diagnostic manufacturers across India and the globe with:
Ready-to-use solutions: ELISA kits, antibodies, reagents ✅
Custom development: From LFA to recombinant protein production ✅
Trusted manufacturing: ISO-certified, quality-driven infrastructure ✅
Made in India, for the world ✅
Explore our catalogue → www.denovobiolabs.com
Contact us: info@denovobiolabs.com
