Meeting EMA & FDA Guidelines

Introduction — why robust HCP detection matters now
Biologic drugs and biosimilars depend on consistent, safe manufacturing. When E. coli is used as the expression host, residual host cell proteins (HCPs) are one of the most important impurities to detect and control. Regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require product-specific HCP characterization and robust control strategies because undetected HCPs can affect product stability, efficacy, and patient safety. See FDA guidance on impurities and EMA expectations for biopharmaceutical control strategies. (FDA guidance, EMA guidance)
This article explains practical approaches to E. coli HCP detection that align with EMA & FDA expectations, discusses common pitfalls, shares how deNOVO Biolabs supports manufacturers with validated reagent solutions, and provides a clear roadmap to regulatory-ready data.
Regulatory context
what EMA & FDA expect for HCP testing?
Both EMA and FDA emphasize product-specific evaluation of HCP risk and control. There is no single numeric acceptance limit that fits every product. Instead, regulators want to see a well-justified strategy that demonstrates HCP detection methods are sensitive, specific, and suitable for the product and intended clinical application.
Key regulatory expectations include:
- Product-specific assay validation: Demonstrate accuracy, precision, specificity, limit of detection (LOD), limit of quantitation (LOQ), linearity, and robustness. (See FDA bioanalytical method validation guidances and EMA quality guidelines.)
- Characterization of HCP profile: Use orthogonal methods where appropriate (ELISA, mass spectrometry) to understand HCP identity and risk.
- Demonstration of removal and process consistency: Show batch-to-batch comparability and process capability for acceptable HCP levels.
- Risk-based acceptance criteria: Justify acceptance limits using clinical risk, dose, route of administration, and patient population.
For full regulator text and recommendations, consult the FDA and EMA guidance libraries: FDA Regulatory Guidance and EMA Scientific Guidelines.
Common analytical approaches for E. coli HCP detection
No single technique is sufficient on its own. Industry practice combines immunoassays (most commonly ELISA) with orthogonal orthogonal analytical methods such as LC-MS/MS for identity and deep profiling.
ELISA — the industry workhorse
ELISA remains the standard for routine HCP quantitation because of sensitivity, throughput, and ease of use. But ELISA performance depends heavily on the antibody reagents and reference standards used. A well-designed anti-E. coli HCP ELISA should be:
- Raised against a representative HCP immunogen (ideally from the same host strain / process),
- Validated for matrix effects (drug interference, high product concentrations),
- Demonstrated for linearity, recovery, and precision across the expected concentration range.
deNOVO’s E. coli HCP ELISA kit, for example, is developed with comprehensive immunogens and validated for a dynamic range suitable for many bioprocess applications, delivering sensitivity down to low ng/mL levels — a performance that matches regulatory expectations for detection capability.
Mass spectrometry (LC-MS/MS)
While ELISA quantifies total HCP immunoreactivity, LC-MS/MS enables identification and semi-quantitation of individual HCP species. LC-MS is especially useful for:
- Identifying problematic HCPs that are enzymatically active or immunogenic,
- Confirming removal of specific HCPs after downstream processing steps, and
- Supporting orthogonal evidence where ELISA alone cannot explain a process deviation.
Combining ELISA for quantitative monitoring and LC-MS for identity provides a robust, regulator-friendly testing strategy.
Orthogonal methods & characterization
Additional approaches include 2-D electrophoresis, Western blotting, and functional assays for enzymatic HCPs. Regulators appreciate a multi-method characterization approach that demonstrates an understanding of both abundance and biological risk.
Practical validation roadmap for HCP ELISA
Here’s a stepwise validation blueprint that maps to regulatory expectations and practical lab implementation:
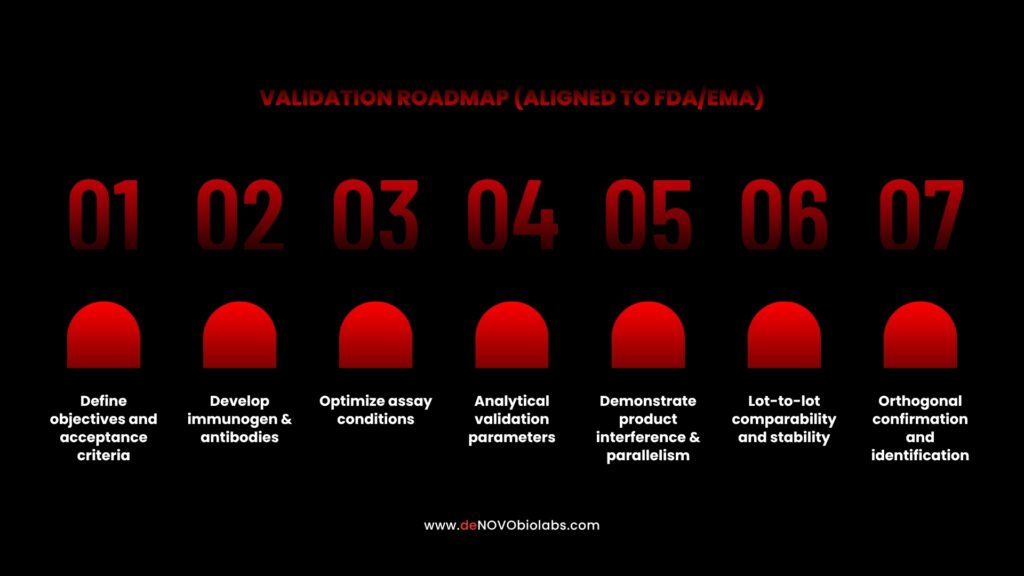
1. Define objectives and acceptance criteria
- Clarify the assay’s purpose: in-process monitoring, final product release, or comparability studies.
- Determine acceptance limits based on process capability and clinical risk rationale.
Regulators favor justified limits over arbitrary cutoffs.
2. Develop immunogen & antibodies
- Use HCP preparations representative of the host strain and process. deNOVO raises antibodies against diverse E. coli HCP pools and strains to maximize coverage across common production platforms.
- Consider both polyclonal and monoclonal strategies: polyclonals can provide breadth, while monoclonals (or optimized monoclonal mixtures) offer specificity and reproducibility for regulated assays.
3. Optimize assay conditions
- Titrate capture and detection antibodies, optimize blocking buffers, and assess incubation times and temperatures.
- Perform spike-and-recovery in relevant matrices (product buffer, serum, etc.) to assess matrix effects.
4. Analytical validation parameters
- Evaluate specificity (no cross-reactivity with product), sensitivity (LOD/LOQ), precision (intra/inter-assay CVs), accuracy (recovery), linearity, and robustness (operator, reagent lot, instrument variation).
- Document system suitability criteria for routine runs.
5. Demonstrate product interference & parallelism
- Establish that high concentrations of drug substance do not mask HCP detection (drug interference).
- Demonstrate parallelism between the standard curve and diluted sample responses where appropriate.
6. Lot-to-lot comparability and stability
- Test multiple production lots to confirm consistent HCP recovery.
- Run accelerated and real-time stability studies on reference standards and antibody reagents.
7. Orthogonal confirmation and identification
- Use LC-MS/MS to identify high-abundance or problematic HCPs. Correlate MS-based identity data with ELISA quantitation to provide a complete picture.
Common pitfalls & how to avoid them
Pitfall #1
Non-representative immunogen
If the antibody is raised against a non-representative or denatured HCP mix, assay coverage will be poor in production samples. Mitigation: use HCP immunogens derived from the actual production strain and process conditions or pooled HCPs reflecting process variability.
Pitfall #2
Matrix suppression and drug interference
High product concentrations or excipients can mask HCP signals. Mitigation: Validate spike-and-recovery across relevant matrices and include sample dilution or product removal steps when needed.
Pitfall #3
Overreliance on a single method
ELISA alone may miss low-abundance but biologically significant HCPs. Mitigation: incorporate LC-MS/MS profiling and functional assays based on risk assessment.
Pitfall #4
Poor reagent traceability
Lot variability in antibodies or standards leads to drift. Mitigation: adopt recombinant antigens, monoclonal antibodies, and strict lot qualification procedures; maintain Certificates of Analysis and robust QC records.
Case example
aligning an HCP assay to EMA/FDA expectations (deNOVO approach)
A mid-sized biopharma company producing a recombinant protein in E. coli approached deNOVO after their in-house HCP ELISA showed inconsistent recovery across batches and poor correlation with MS data.
deNOVO’s approach:
- Performed HCP immunogen design using pooled E. coli extracts from multiple production runs.
- Developed a mixed monoclonal/polyclonal reagent set to maximize coverage while preserving reproducibility.
- Performed a full validation:
sensitivity (LOD 1–5 ng/mL depending on matrix)
precision (intra-assay CV <10%, inter-assay CV <20%)
recovery (85–115%)
and matrix robustness testing. - Correlated ELISA results with LC-MS/MS profiles to identify a small set of residual enzymatic HCPs that required process optimization.
Outcome: the company achieved consistent HCP detection across lots, better correlation with orthogonal MS data, and a well-documented validation package acceptable to both internal QA and external regulatory reviewers.
How reagent strategy affects long-term compliance?
Regulatory reviewers focus less on the brand and more on traceability, validation, and reproducibility. Reagent strategy matters:
- Polyclonal antibodies provide broad coverage but can show lot variability. They are useful for early screening and initial process understanding.
- Monoclonal antibodies and recombinant antibodies provide defined sequence identity and consistent production — ideal for release testing and long-term supply.
- Recombinant antigen standards reduce variability in quantitation and support robust standard curves.
deNOVO supplies reagent packages (antibodies + standards) with complete documentation, lot comparability data, and support for regulatory submissions.
Practical checklist
what to include in a regulator-ready HCP dossier?
When compiling documentation, ensure you include:

Providing this level of documentation shortens review cycles and raises confidence with regulatory assessors.
deNOVO capabilities
how we support HCP detection and regulatory readiness?
deNOVO Biolabs offers an integrated HCP support package for E. coli systems:
- Custom HCP immunogen preparation using process-representative extracts
- Antibody generation (polyclonal, monoclonal, recombinant) with epitope coverage mapping
- Validated ELISA kits for E. coli HCP detection with documented LOD/LOQ and dynamic range
- LC-MS/MS profiling support through validated partner labs (identity confirmation and targeted monitoring)
- Analytical validation services and data packages tailored to EMA/FDA submission formats
- Technical consulting for assay design, product interference mitigation, and acceptance criteria justification
If you need end-to-end support — from immunogen design to regulatory dossier — deNOVO can deliver reagents, validation, and documentation that align with agency expectations.
Need a technical review of your HCP assay or a feasibility plan for replacing unstable reagents?
Book a free feasibility call with deNOVO’s HCP team
Contact deNOVO Biolabs
Emerging trends & future directions in HCP detection
1. Deeper LC-MS quantitation
LC-MS is improving in sensitivity and quantitation, enabling better identification and targeted monitoring of critical HCPs.
2. Recombinant and sequence-defined reagents
Recombinant antibodies and sequence-defined standards reduce uncertainty and simplify lot qualification.
3. Data integration & digital QC
Linking ELISA runs, MS profiles, and process parameters in LIMS and dashboarding tools improves trend detection and process control.
4. Risk-based regulatory dialogue
Regulators increasingly accept risk-based approaches; presenting a clear risk assessment backed by orthogonal analytical evidence speeds acceptance.
Conclusion
Detecting E. coli HCPs to the standards expected by EMA & FDA requires a thoughtful, product-specific approach. ELISA remains central, but it must be supported by representative immunogens, validated reagents, orthogonal identity methods, and rigorous documentation. Reagent strategy — particularly the use of well-characterized monoclonal or recombinant antibodies and recombinant standards — is a decisive factor in long-term assay reliability.
At deNOVO Biolabs, we partner with manufacturers to build assays and dossiers that pass regulatory and operational scrutiny. If you are preparing a submission, optimizing your release testing, or need a robust HCP monitoring strategy, we can help with reagents, validation, and regulatory-ready documentation.
Get a free assessment of your HCP testing strategy and a customized validation plan — contact our HCP experts today:
Book a technical call →
