
In today’s fast-moving biopharma landscape, speed and reliability are non-negotiable. The ability to go from gene → expression → purification → functional protein is the foundation of drug discovery, diagnostics, and biologics manufacturing. But each of these stages brings technical challenges, validation needs, and risk.
That is why end-to-end gene & protein platforms—where a provider supports every step—are becoming critical. In this post, we will explore:
- The importance of integrated gene-to-protein workflows
- Trends and market drivers for recombinant proteins
- Key technical stages (gene design, expression, purification, validation)
- Challenges and best practices
- Capability highlights from deNOVO Biolabs
- How such platforms can accelerate your pipeline
- A practical CTA for engaging with deNOVO
The Market
- The global biopharmaceutical market was valued at about USD 452 billion in 2024 and is projected to grow rapidly. Grand View Research
- The recombinant proteins market alone was estimated at USD 2.3 billion in 2023, with a projected CAGR of ~7.1% through 2032. Global Market Insights Inc.
- As biologics, cell therapies, and diagnostics rise, the demand for robust, scalable protein platforms increases.
These numbers reflect that biopharma is not just about drug targets—it is about the tools that enable them.
Why “End-to-End” is crucial?
Disparate service providers often leave biopharma teams juggling multiple vendors—each requiring handoffs, extra validation, integration risk, and communication overhead. An end-to-end provider:
- Enables data continuity, reducing errors from format changes
- Ensures validated interfaces between stages (e.g. gene → vector → expression)
- Maintains accountability across the full chain
- Allows faster troubleshooting since one team owns the entire flow
That consistency becomes a competitive edge in high-stakes biologics development.
Key Stages in Gene-to-Protein Workflow
Let’s walk through each step and the challenges / best practices:
1. Gene Design & Optimization
- Start from target sequence (e.g. human antibody gene, cytokine, enzyme)
- Codon optimization for your host (E. coli, CHO, yeast)
- Removal of problematic motifs (e.g. cryptic splice sites, repeats)
- Addition of tags, signal peptides, secretion signals
Best practice: Use in silico tools to predict mRNA folding, translation efficiency, and removal of low-complexity regions to avoid expression bottlenecks.
2. Cloning & Vector Construction
- Insert the optimized gene into an expression plasmid
- Choose appropriate promoters, enhancers, selectable markers
- Validate sequence integrity with Sanger or NGS
Challenge: Plasmid stability under production conditions, vector backbone interactions.
3. Expression / Host System
- Choose host (E. coli, CHO, yeast, insect) based on protein properties
- Optimize culture conditions: temperature, feed, induction timing
- Monitor yield vs. quality trade-offs
Note: The recombinant proteins market is expected to grow sharply as demand for therapeutics and diagnostics increases. BioSpace
4. Purification & Downstream Processing
- Common steps: affinity chromatography, ion exchange, size exclusion
- Use endotoxin removal (especially for E. coli systems)
- Monitor purity, yield, aggregation, host cell impurities
Best practice: Multi-step purification pipelines and orthogonal QC to separate product from impurities.
5. Characterization & Validation
- Structural validation: SDS-PAGE, Western blot, mass spectrometry
- Functional assays: ELISA, binding kinetics (e.g. SPR), enzymatic activity
- Stability studies, batch-to-batch reproducibility
6. Formulation & Delivery
- Buffer optimization, excipients, stability under storage
- For diagnostics, conjugation or labeling (e.g., for lateral flow assays)
- Packaging, sterile filtration, QC release
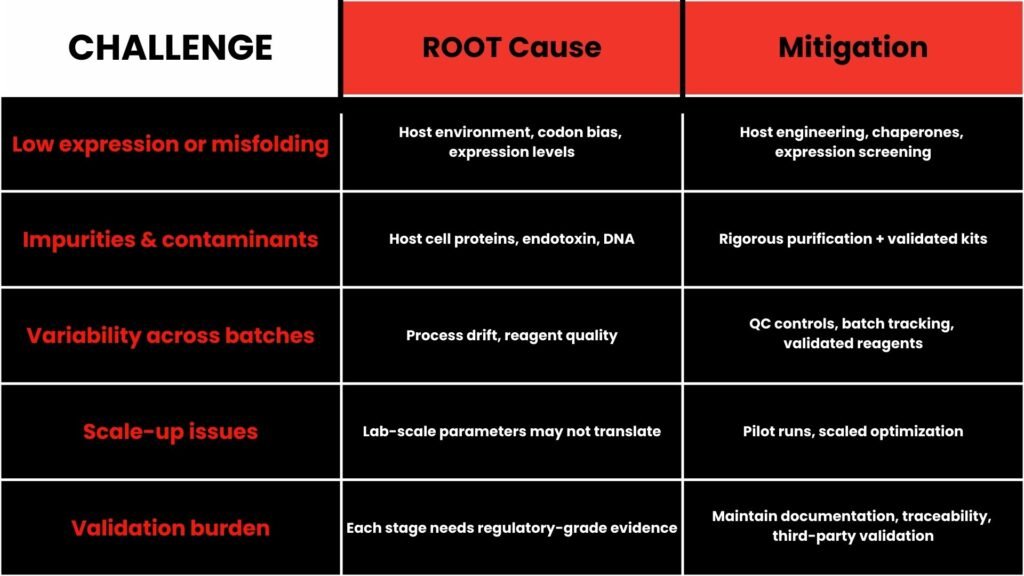
Challenges & Mitigation in Gene-to-Protein
deNOVO Biolabs’ Capabilities in Gene-to-Protein
At deNOVO, we have built a suite of capabilities to support your pipeline end-to-end:
- Gene optimization and vector design services tailored for your target host
- Recombinant protein expression in bacterial, mammalian, or hybrid systems
- High-purity purification & QC workflows with validated reagents
- Protein characterization, functional assays, and validation support
- Custom solutions for diagnostic antigen, therapeutic candidates, antibody fragments
We combine scientific rigor with operational reliability so you can focus on your therapeutic or diagnostic target—not on vendor handoffs.
Use Cases
- Diagnostics companies building antigen reagents find that having a single provider from gene → protein saves months in troubleshooting.
- Biologics developers use deNOVO’s protein modules to prototype leads faster, validate candidates in vivo, and scale bench-to-batch more confidently.
- CRO collaborations where consistent reagent supply and reproducibility are essential for contract deliverables.
How to choose a Gene-to-Protein partner?
Look for providers that offer:
- Transparent validation data and reproducibility reports
- Scalability from mg to gram levels
- Documentation suited for regulatory submission
- Scientific support and iterative optimization
- Flexibility across host systems
At deNOVO, those are not add-ons—they are core to our offering.
If your team is developing biologics, diagnostic proteins, or assay reagents and you’re seeking a trusted gene-to-protein partner, let’s talk.
We’d love to understand your target antigen or therapeutic, and show how our integrated platform can accelerate your path.
Connect with us via our contact page or drop us a message to explore collaboration.

Pingback: The Hidden Science behind Reliable Diagnostics: Recombinant Proteins - deNOVO Biolabs
binance
7 Feb 2026Thanks for sharing. I read many of your blog posts, cool, your blog is very good.