This is how ‘Native CRP Protein’ and ‘HBsAg Ad/Ay Antigens’ are powering diagnostics
Introduction
In the diagnostic landscape, precision starts with high-quality reagents. Two critical components, C-reactive protein (CRP) for inflammation assays, and HBsAg Ad/Ay antigens for hepatitis B detection are key to developing sensitive, reliable tests capable of delivering accurate results across diverse real-world environments.
In this blog, we will explore:
- Why native CRP and HBsAg Ad/Ay antigens matter?
- Their role in current diagnostic markets
- How they support assay reliability?
- Why deNOVO Biolabs is a trusted partner?
1. Why is the importance of Native CRP Protein?
CRP is an acute-phase protein produced by the liver in response to inflammation—and it’s a widely adopted biomarker for everything from infection to cardiovascular risk.
- High-sensitivity CRP (hs-CRP) assays are now an industry standard, especially for evaluating cardiovascular disease risk. In 2024, hs-CRP held nearly 42–43% of the total CRP testing market share globally (Grand View Research, Market.us).
- The broader global CRP testing market is projected to surge from $2.28 billion in 2023 to $4.17 billion by 2034, growing at a CAGR of approximately 5.6% (Research and Markets).
High-quality native CRP protein ensures consistent calibration, enabling:
- Accurate quantification across immunoassay types (ELISA, immunoturbidimetry, CLIA)
- Stability in point-of-care and central lab workflows
- Reliable detection even at low levels for early disease insight
2. What are HBsAg Ad/Ay Antigens?
Hepatitis B surface antigen (HBsAg) remains the primary marker for HBV detection. It’s particularly important in the transfusion window period—when early detection of acute or chronic HBV infection is crucial. Globally, an estimated 254 million people live with chronic HBV infection, and nearly 820,000 deaths occurred in 2022 due to HBV-related complications (Wikipedia).
Using HBsAg antigens representing both Ad and Ay subtypes ensures:
- Broader assay coverage across diverse patient populations
- Improved sensitivity without compromising specificity
- More reliable detection in field conditions where subtype prevalence can vary
3. Market & Impact
Diagnostics developers are navigating complex environments, from urban hospitals to remote clinics. Reagents that consistently perform across settings can dramatically reduce validation cycles and regulatory risks.
For CRP:
- Immunoturbidimetric assays hold dominant revenue share thanks to speed and cost-effectiveness, benefiting from strong performance reagents (Grand View Research, Research and Markets).
- hs-CRP continues to grow rapidly, especially for cardiovascular risk applications (Market.us, novaoneadvisor.com).
For HBsAg:
- With HBV remaining a global health challenge, accurate and early detection through validated antigens is essential for screening and public health programs (Wikipedia).
4. How deNOVO Biolabs adds value?
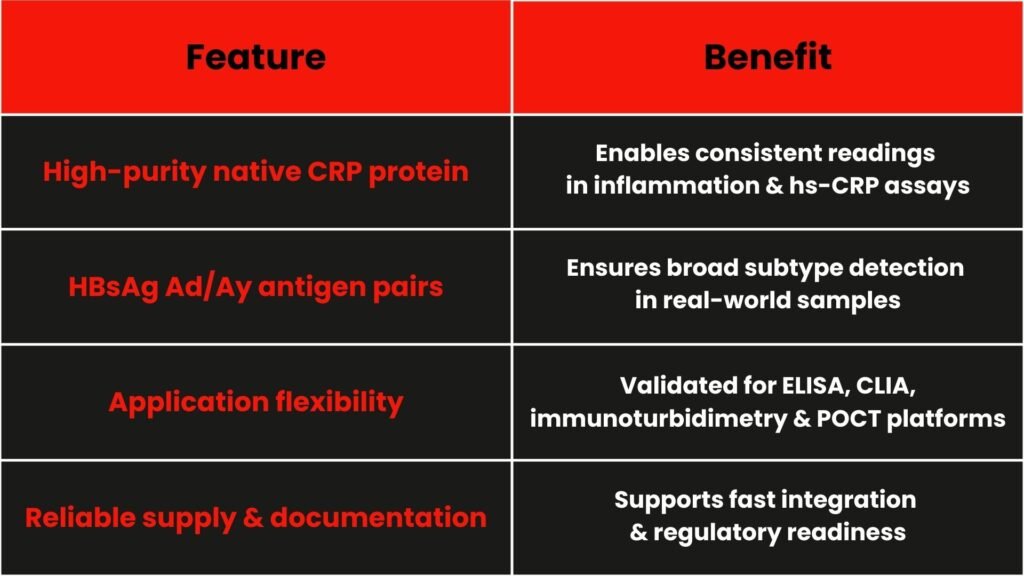
5. What does it means for diagnostic developers?
By choosing validated reagents like our native CRP and HBsAg pairs, you gain:
- Smoother assay development and validation
- Reduced variability across test batches
- Faster movement from concept to regulatory submission
Ready to strengthen your inflammation and hepatitis B diagnostic workflows?
Contact us for consultation and samples—let’s build diagnostics you can truly rely on.

binance napotitev
18 Feb 2026Thanks for sharing. I read many of your blog posts, cool, your blog is very good.