Biosimilar PK ELISA Kits
Biosimilar PK ELISA Kits
Denovo Biolabs has developed enzyme-linked immunosorbent assays (ELISAs) for the pharmacokinetic (PK) monitoring of serum antibody levels.
The ELISA is a well established immunoassay, with clear guidelines for validation when used as a quantitative assay. However, these parameters may not always be relevant for a semi-quantitative assay used to assess whether a sample is positive or negative for a novel marker such as an antibody developed against a therapeutic antibody. We here are in the development of a quantitative PK ELISA and the different approaches of validation to prove each assay which are ‘fit for purpose.’ The parameters of linearity, accuracy, lowest level of detection, intra-assay and inter-assay variability were assessed for the quantitative PK assay. The validation outcome showed that each assay was robust, reliable and accurate to meet the requirements of the intended analytical application, that being to 1) quantitatively determine the concentration of antibody in the serum. Each assay has been successfully translated for use in a clinical trial with adequate quality controls and acceptance criteria set for monitoring consistency and performance.
Denovo Biolabs has developed enzyme-linked immunosorbent assays (ELISAs) for the pharmacokinetic (PK) monitoring of serum antibody levels.
The ELISA is a well established immunoassay, with clear guidelines for validation when used as a quantitative assay. However, these parameters may not always be relevant for a semi-quantitative assay used to assess whether a sample is positive or negative for a novel marker such as an antibody developed against a therapeutic antibody. We here are in the development of a quantitative PK ELISA and the different approaches of validation to prove each assay which are ‘fit for purpose.’ The parameters of linearity, accuracy, lowest level of detection, intra-assay and inter-assay variability were assessed for the quantitative PK assay. The validation outcome showed that each assay was robust, reliable and accurate to meet the requirements of the intended analytical application, that being to 1) quantitatively determine the concentration of antibody in the serum. Each assay has been successfully translated for use in a clinical trial with adequate quality controls and acceptance criteria set for monitoring consistency and performance.
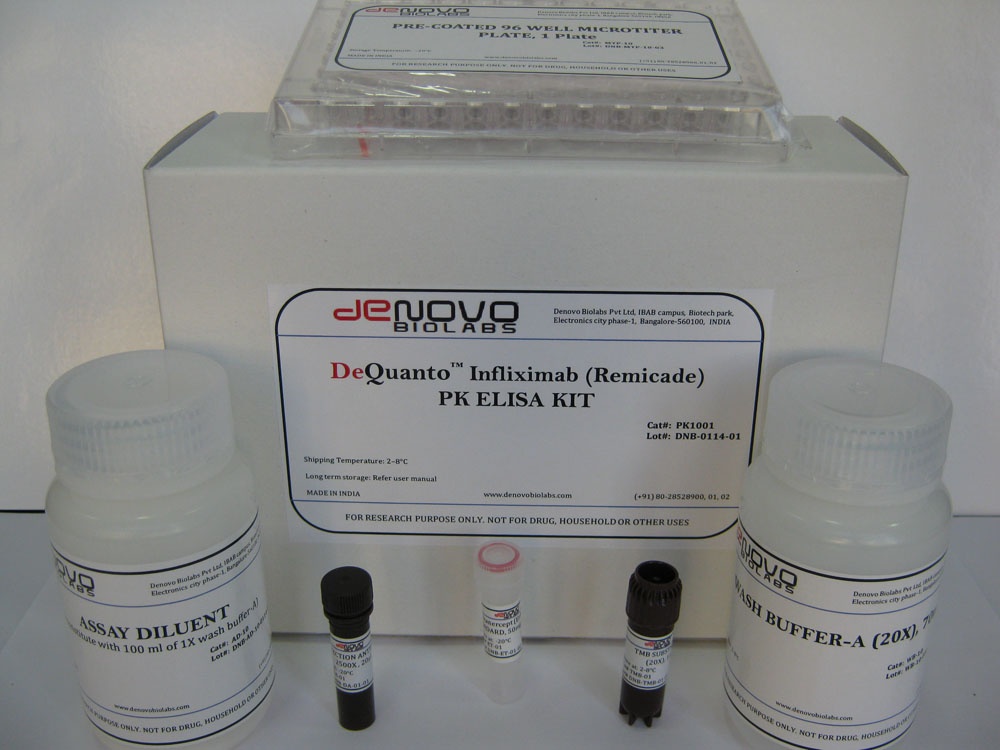
- DeQuantoTM Infliximab (Remicade®) PK ELISA KIT (Catalog # PK1001)
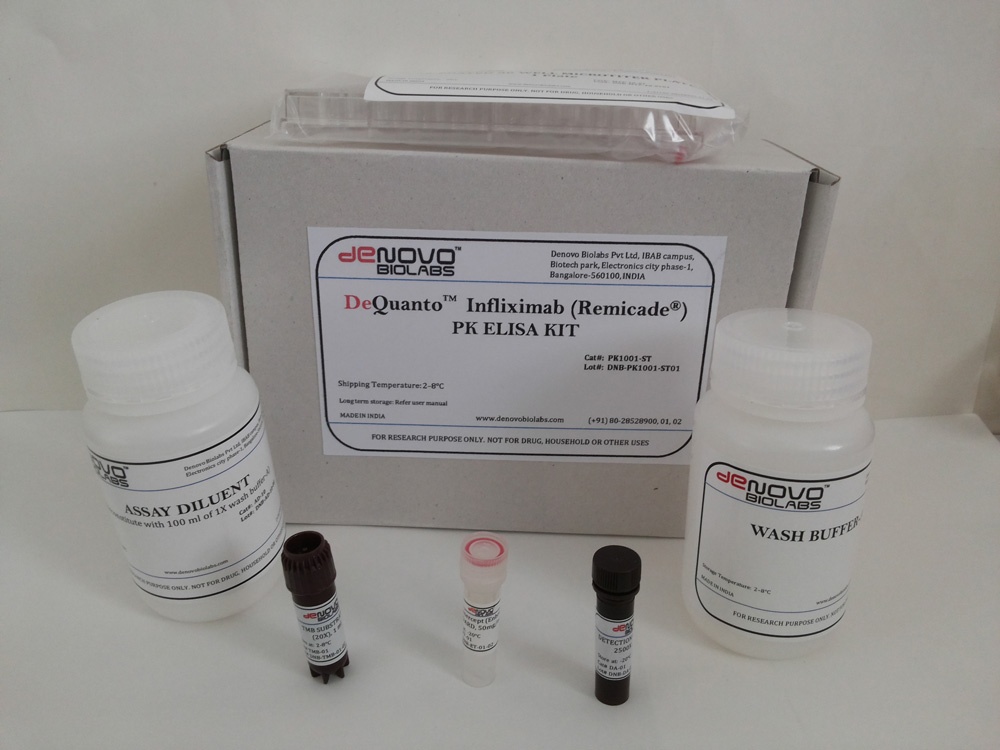
- DeQuantoTM Infliximab ( Remicade®) PK ELISA KIT (Catalog # PK1001-ST)
- DeQuantoTM Adalimumab (Humira®) PK ELISA KIT (Catalog # PK1002)
- DeQuantoTM Adalimumab (Humira®) PK ELISA KIT (Catalog # PK1002-ST)
- DeQuantoTM Etanercept (Enbrel®) PK ELISA KIT (Catalog # PK1003)
- DeQuantoTM Etanercept (Enbrel®) PK ELISA KIT (Catalog # PK1003-ST)
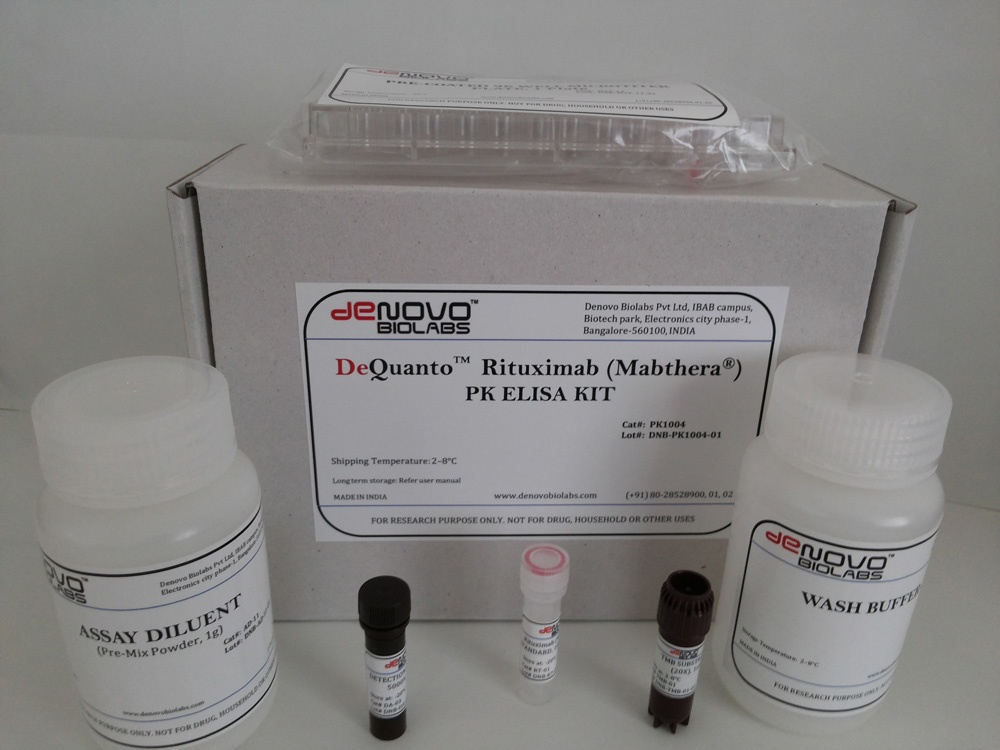
- DeQuantoTM Rituximab (Mabthera®) PK ELISA KIT (Catalog # PK1004)
- DeQuantoTM Bevacizumab (Avastin®) PK ELISA KIT (Catalog # PK1005)
- DeQuantoTM Ranibizumab (Lucentis®) PK ELISA KIT (Catalog # PK1006)
- DeQuantoTM Trastuzumab (Herceptin®) PK ELISA KIT (Catalog # PK1007) (Upcoming)
01
DeQuantoTM Infliximab (Remicade®) PK ELISA KIT (Catalog # PK1001)
02
DeQuantoTM Infliximab ( Remicade®) PK ELISA KIT (Catalog # PK1001-ST)
03
PK1002)
04
DeQuantoTM Adalimumab (Humira®) PK ELISA KIT (Catalog # PK1002-ST)
05
DeQuantoTM Etanercept (Enbrel®) PK ELISA KIT (Catalog # PK1003)
06
PK1003-ST)
07
DeQuantoTM Rituximab (Mabthera®) PK ELISA KIT (Catalog # PK1004)
08
PK1005)
09
DeQuantoTM Ranibizumab (Lucentis®) PK ELISA KIT (Catalog # PK1006)
10
PK1007) (Upcoming)
| Catalouge No | Product | Assay Matrix | Assay Range | Sensitivity | Storage |
|---|---|---|---|---|---|
| PK1001 | DeQuanto® Infliximab (Remicade®) PK ELISA KIT | Human serum and plasma | 6.58-50 ng/ml | 6 ng/ml |
Shipped at 2-8°C. Longterm Storage at -20° C |
| PK1002 | DeQuanto® Adalimumab (Humira®) PK ELISA KIT | Human serum and plasma | 5.2 – 200 ng/ml | 5.2 ng/ml |
Shipped at 2-8°C. Longterm Storage at -20° C |
| PK1003 | DeQuanto® Etanercept (Enbrel®)PK ELISA KIT | Human serum and plasma | 300 ng/ml to 2.34 ng/ml | 2.34 ng/ml |
Shipped at 2-8°C. Longterm Storage at -20° C |
| PK1004 | DeQuanto® Rituximab (Mabthera®) PK ELISA KIT | Human serum and plasma | 6.25-200 ng/ml | 6.25 ng/ml |
Shipped at 2-8°C. Longterm Storage at -20° C |
| PK1005 | DeQuanto® Bevacizumab (Avastin®) PK ELISA KIT | Human serum and plasma | 0.1 – 3.1 ug/ml | 1.17 ng/ml |
Shipped at 2-8°C. Longterm Storage at -20° C |
| PK1006 | DeQuanto® Ranibizumab (Lucentis®) PK ELISA KIT | Human serum and plasma | 1250 – 9.77 ng/ml | 4.88ng/ml |
Shipped at 2-8°C. Longterm Storage at -20° C |
| PK1009 | DeQuanto® Denosumab (Xgeva®) PK ELISA KIT | Human serum and plasma | 1.95 – 1000 ng/ml | 3.91 ng/ml |
Shipped at 2-8°C. Longterm Storage at -20° C |
| IM5004 | DeQuanto® Rituximab (Mabthera®) Immunogenicity PK ELISA KIT | Human serum and plasma | refer cut off values | refer cut off values |
Shipped at 2-8°C. Longterm Storage at -20° C |
| IM5005 | DeQuanto® Adalimumab (Humira®) Immunogenicity PK ELISA KIT | Human serum and plasma | refer cut off values | refer cut off values |
Shipped at 2-8°C. Longterm Storage at -20° C |
| IM5006 | DeQuanto® Ranibizumab (Lucentis®) Immunogenicity PK ELISA KIT | Human serum and plasma | refer cut off values | refer cut off values |
Shipped at 2-8°C. Longterm Storage at -20° C |