When it comes to antibody development — whether for diagnostics, therapeutics, or research — purity, specificity, and consistency are key. One of the most powerful methods to elevate antibody performance is antigen affinity purification. This technique uses the very antigen to “fish out” high-quality antibodies that bind it strongly, significantly improving assay sensitivity, reducing background noise, and boosting reproducibility.
In this blog, you will learn:
- What antigen affinity purification is (and how it works)?
- Why it matters — how it improves antibody performance?
- Practical design choices, pros/cons, and pitfalls
- How deNOVO Biolabs applies it in our offerings?
- Use cases, tips, and how you can work with deNOVO
Let’s explore how antigen affinity purification can transform your antibody workflows.
What is Antigen Affinity Purification?
Affinity purification is a variant of chromatography where a specific ligand is immobilized on a solid support to selectively bind the target molecule. In antigen affinity purification, the antigen (or epitope) is used as the ligand to capture antibodies that specifically bind that antigen. This allows you to enrich only those antibodies that recognize your specific antigen — discarding unrelated immunoglobulins. (Thermo Fisher Scientific)
Here is a simplified workflow:
- Antigen immobilization
The antigen (often recombinant, purified) is covalently attached to a resin or bead (agarose, magnetic beads, or specially activated matrices). (bioclone.net) - Binding / incubation
The antibody-containing sample (serum, hybridoma supernatant, culture medium) is passed over the antigen-ligand support under optimal buffer conditions so specific antibodies bind. - Washing
Non-specific proteins and weakly bound antibodies are washed away using suitable buffers (with salt, detergents, pH control) to reduce background. - Elution
Bound antigen-specific antibodies are then released (typically by a change in pH, ionic strength, or using competitive elution) and collected. Immediate neutralization is needed to preserve antibody integrity. (agrisera.com) - Polishing / buffer exchange
The eluted antibodies may be further cleaned (e.g., via desalting or size-exclusion) and formulated for storage or downstream assays.
Because antigen affinity purification exploits the specific binding interface, it results in highly selective enrichment of the most relevant antibodies.
Why Antigen Affinity Purification
enhances antibody performance?
Using antigen affinity purification offers multiple tangible advantages that translate to better performance in assays, diagnostics, and research:
1. Higher specificity, lower background
Since only antigen-binding antibodies are retained, non-specific immunoglobulins and serum proteins are largely eliminated. The result: lower background noise and cleaner signals in ELISA, Western blot, immunohistochemistry, etc. (seracare.com)
2. Improved sensitivity
By enriching the “true binders,” you increase the fraction of high affinity antibodies in your preparation. This can allow detection of lower antigen concentrations. In diagnostic assays, every reduction in detection limit (e.g. from 5 ng/mL to 1 ng/mL) can make a difference in early detection.
3. Better reproducibility & consistency
Because the purification is based on the antigen you care about, each batch has more uniform binding behavior. This reduces lot-to-lot variability and enhances the robustness of downstream assays.
4. Enhanced assay pairing flexibility
You can use antigen-purified antibodies both as capture and detection reagents (in sandwich ELISA), because you have selected for antibodies that truly bind the target antigen. (seracare.com)
5. Removal of cross-reactive species
In some workflows, negative affinity steps can be used to subtract antibodies that cross-react with unwanted antigens, further increasing specificity at the cost of potentially trimming sensitivity. (seracare.com)
A review of antibody purification methods states that antigen affinity purification is often considered the “purest” method, producing antibodies with minimal cross-reactivity and maximal specificity. (seracare.com)
Design Considerations
While antigen affinity purification is powerful, its success depends on thoughtful design. Here are key parameters and trade-offs to consider:
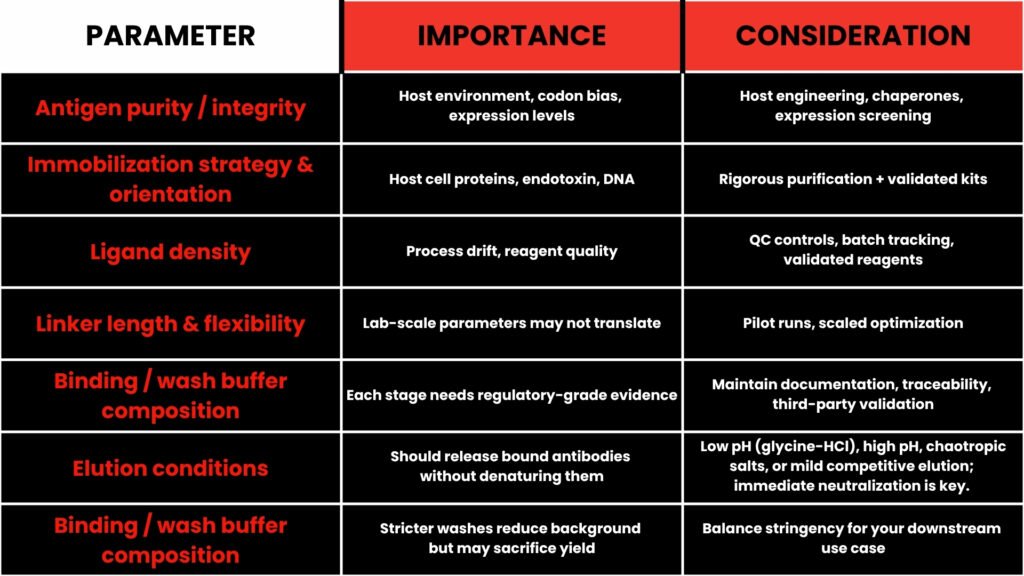
A caution: harsh elution conditions (e.g. extreme pH, chaotropic agents) may damage antibody activity or antigen-binding sites. Always test and validate across conditions. (agrisera.com)
How we leverage Antigen Affinity Purification?
At deNOVO Biolabs, we harness antigen affinity purification as a core pillar in designing high-quality antibodies and diagnostic reagents. Here’s how we apply it:
- Custom antigen-linked resins
We immobilize recombinant antigens (designed for your application) onto optimized resins or magnetic beads, ensuring epitope accessibility and binding strength. - Selective enrichment of high-performance antibodies
During screening and production, we refine polyclonal or monoclonal antibody pools by antigen affinity purification to enrich the highest binding species. - Low background & high sensitivity reagents
Because of the antigen-based selection, the antibodies we deliver show minimal non-specific noise and enhanced assay sensitivity. - Kit-grade performance & consistency
Our purified antibodies are validated batch-wise for consistent binding kinetics, stability, and performance in sandwich ELISA or other immunoassays. - Supporting custom assay development
When clients require tailored detection systems, we integrate antigen affinity purification in our workflow (immunization → screening → purification → assay development). This contributes to faster timelines (6–8 months typical) and robust final products.
When you partner with deNOVO, you are not just getting an antibody — you’re getting a reagent refined using best-in-class purification to deliver performance and reliability.
Practical Scenarios
Here are example scenarios where antigen affinity purification can make a real difference:
- Sandwich ELISA for low-abundance biomarkers
To detect trace analytes, the capture and detection antibodies both need exquisite specificity. Affinity purification ensures your antibodies won’t pull along irrelevant IgGs that raise background. - Competition assays or inhibition assays
In these formats, cross-reactivity or non-specific binding can distort results. Purified antibodies maintain signal fidelity. - Western blot / immunoprecipitation
Antigen-purified antibodies reduce off-target bands or pull-downs, enabling cleaner blots. - Therapeutic antibody characterization / binding kinetics
When measuring KD / off-rates, you want your sample free from interfering immunoglobulins. - Multiplexed assays / multiplex immunoassays
Minimizing cross-reactivity is critical; affinity purification helps eliminate unwanted cross-binding.
In short: any application where specificity, sensitivity, and reproducibility matter — antigen affinity purification is a valuable tool.
Challenges, Mitigations & Best Practices
While powerful, antigen affinity purification has challenges. Here are common ones and how to address them:
- Antigen denaturation during immobilization
Use milder coupling chemistries (e.g., gentle crosslinkers, oriented coupling) to preserve conformation. - Poor recovery or low yield
Optimize elution (mild buffer adjustments, shorter exposure) and prevent antibody degradation (immediate neutralization). - Epitope masking / steric hindrance
Use spacer arms or orient antigen coupling to expose binding sites. - Cross-reactive species not removed
Add negative affinity subtraction columns using known off-target antigens to subtract cross-reactors. (seracare.com) - Batch-to-batch variability
Standardize coupling density, buffer conditions, and characterization assays (e.g. binding kinetics, validation runs). - Cost & time investment
Affinity purification is more expensive than generic IgG purification. But the pay-off in assay performance often justifies the investment.
Applying these best practices ensures that antigen affinity purification delivers real, scalable benefits rather than just theoretical ones.
If your team is working on diagnostic assay development, immunoassays, antibody engineering, or biomarker measurement — let’s talk. At deNOVO Biolabs, we offer:
- Ready-to-use antigen-purified antibodies
- Custom antigen affinity purification workflows
- Integrated antibody development + assay design
- Validation support & performance benchmarking
👉 Request a feasibility conversation or pilot evaluation with us.
Drop a message to info@denovobiolabs.com or visit our website’s “Contact Us” page.
When you partner with deNOVO, you gain access to purification strategies honed for diagnostics, reproducibility, and regulatory readiness.
