
Introduction
When producing biotherapeutics—monoclonal antibodies, fusion proteins, or other recombinant products—the cells that make them matter almost as much as the product itself. In mammalian expression systems, CHO (Chinese Hamster Ovary) cells are by far the most commonly used. Yet, along with the desired protein, CHO cells secrete or retain a variety of host cell proteins (HCPs).
Residual CHO HCPs are process-related impurities. If not adequately detected and removed, they can affect product stability, safety, immunogenicity, and regulatory compliance. This blog explains:
- What CHO HCPs are and why they matter?
- Current regulatory & industry standards on HCP levels
- Key analytical methods (especially ELISA) to monitor HCP clearance
- Common challenges and how manufacturers address them
- How deNOVO’s DeQuanto® E. coli HCP ELISA Kit (12×8 wells) fits into this landscape for companies also using bacterial systems, or for complementing CHO-HCP efforts?
By the end, you will understand not only why CHO HCP quantification is non-negotiable, but also what tools & practices help you do it well.
What are CHO HCPs and why they matter?
What are they?
- During upstream production (cell culture), CHO cells naturally produce thousands of intracellular and secreted proteins. Some are part of normal cell metabolism; others are stress-induced or byproducts. These are collectively called CHO host cell proteins.
- When downstream purification isn’t perfect, some HCPs “co-purify” with the therapeutic protein, partly due to similar physical/chemical properties, or because they bind to the product (either directly or via aggregates).
Why They Matter?
- Immunogenicity risk & safety
Some CHO HCPs can trigger immune responses in patients. Regulatory bodies increasingly demand rigorous assessment of this risk. For example, ISPRI-HCP is a 26-company FDA-supported initiative targeting the immunogenic potential of numerous CHO HCPs. (U.S. Food and Drug Administration) - Product stability & potency
HCPs may degrade product, affect formulation, or interfere with activity. Proteases leftover from CHO cells can degrade the therapeutic protein over storage. - Regulatory compliance & batch release
Regulatory guidelines (e.g., FDA, EMA, ICH) view residual HCP as a critical quality attribute (CQA). Before clinical trials or commercial release, manufacturers must demonstrate that HCP levels are consistent, sufficiently low, and controlled. - Public perception & quality assurance
High HCP content can create batch recalls, reduce trust, or cause product failures. Even if small in proportion, failures in safety margin can compromise reputation.
Standards, Typical Levels and Risk Assessment
- CHO cells are used to produce nearly 90% of therapeutic monoclonal antibodies (mAbs) and Fc-fusion proteins worldwide. (Nature)
- Regulatory expectations often require residual HCP levels to be below ~100 ppm (parts per million) in many final therapeutic protein products. (Nature)
- Industry studies have shown that in many cases, even residual HCPs present at higher parts per million during intermediate purification aren’t necessarily more immunogenic than purified versions—provided purification is consistent and analytical detection is reliable. (PubMed)
Risk assessment frameworks often include:
- Analytical sensitivity of the assay
- Antibody coverage (does the anti-HCP reagent recognize the spectrum of HCPs present?)
- Immunogenic potential (in silico, in vitro, clinical)
- Batch-to-batch consistency
- Orthogonal methods (mass spectrometry, etc.) to confirm what ELISA shows. (PMC)
Analytical Tools & Methods
ELISA (Total/Generic & Process-Specific)
- Generic HCP ELISAs use polyclonal antibodies raised against CHO HCP mixtures (mock or null cell cultures). They provide broad coverage, useful during early stages of process development. (PerkinElmer Blog)
- Process-specific ELISAs use anti-HCP reagents raised against HCPs from a particular cell line or purification process, offering better specificity in that context.
Advantages of ELISA:
- High throughput, relatively simple workflows
- Sensitivity often down to low ng/mg or ppm levels
- Widely accepted in regulatory settings
Limitations:
- Antibody coverage gaps: some HCPs may evade detection if not immunoreactive, or due to epitope masking. (PubMed)
- Variability: upstream process changes (culture media, expression system, temperature, etc.) can change HCP profiles. (Cytiva)
Orthogonal Methods: MS, Western Blot, etc.
- Mass spectrometry (LC-MS/MS) can identify and quantify individual HCP species, even those at low abundance or weakly immunogenic. (Nature)
- 2D electrophoresis / Western Blot help in mapping coverage and verifying that ELISA reagents detect HCP species.
Common Challenges in CHO HCP Quantification & How to Overcome them?
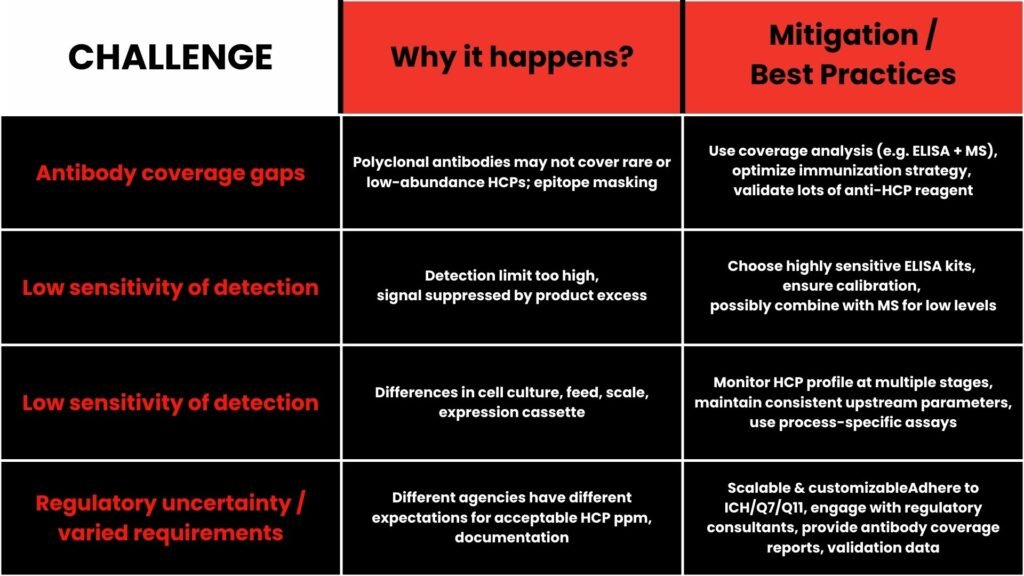
deNOVO’s Capability &
the Role of DeQuanto® E. coli HCP ELISA Kit
While much of the HCP discussion centers on CHO systems, many biotherapeutics (especially for diagnostic reagent production, microbial expressions, or certain proteins) use E. coli as the host. E. coli HCPs pose similar but distinct challenges.
Here’s how deNOVO supports biopharma & diagnostics manufacturers:
- DeQuanto® E. coli HCP ELISA Kit (12×8 wells — a reliable kit to quantify E. coli host cell proteins. Designed for sensitivity, specificity, and to support process validation.
- Quality-controlled reagents, consistent batches, and detailed documentation ensure you can trust what you measure.
- Although this kit is for E. coli systems, the principles translate: good antibody coverage, detection sensitivity, and validation.
If your facility uses CHO or E. coli systems—or both—robust HCP monitoring (using ELISA + orthogonal confirmation) is essential. deNOVO can help you integrate reliable solutions into your QC, process development and regulatory submission workflows.
Case Studies / Examples
- Industry Study on HCP ELISA Limitations:
A study by Zhu-Shimoni et al. showed that generic HCP ELISAs, though powerful, have limitations: weakly immunoreactive proteins or antigen excess can mask or distort measurement. (PubMed) - Recent Discovery of Microprotein Impurities:
Research in 2024 revealed that almost 90% of therapeutic mAbs made in CHO carry micro-protein impurities that are not part of standard annotations, affecting detection and possibly safety/protein stability. (Nature) - Risk Prediction Tools:
ISPRI-HCP and similar immunoinformatics tools are now used to assess “high-risk” HCPs by epitope density and similarity to human sequences. They highlight that while many CHO HCPs exist, only some have immunogenic potential. (SpringerLink)

Regulatory Considerations
- Regulatory bodies expect manufacturers to demonstrate that residual HCP is controlled and reliably quantified.
- USP, ICH, EMA, and FDA provide guidelines and expectations for HCP as part of CQAs.
- Product release criteria often include HCP ppm thresholds, acceptable variation, identity of risky HCPs.
- During biosimilar development, difference in HCP profile compared to innovator product can be scrutinized.
Why deNOVO and DeQuanto® Kit Are a Smart Investment?
- Although DeQuanto® is for E. coli HCP, using similar validated kits in your purification pipeline reduces risk, speeds up validation, and improves product consistency.
- For CHO systems, having a partner that understands HCP monitoring, offers support, and provides validated reagents, is crucial.
If you are a biopharma R&D scientist, QC leader, or diagnostic manufacturer, ask yourself:
- Do your CHO HCP levels meet industry regulatory expectations?
- Are your ELISA reagents well-validated and with good antibody coverage?
- Could integrating more sensitive HCP quantification tools save you time, reduce failures, or improve safety?
Let’s connect. deNOVO Biolabs offers validated kits, expert reagent development, and technical support to help you meet your HCP challenges with confidence.
📩 Reach out via our contact page → [www.denovobiolabs.com/contact]
Learn more about DeQuanto® and our ELISA kits for HCP quantification.
